ESGLI
Evaluation of 16 urinary antigen tests for Legionella pneumophila serogroup 1 LPS detection
Click here to download the study
Downloads
INTRODUCTION
Legionnaires’ disease (LD), caused by Legionella pneumophila represent 2-8% of community-acquired pneumonia with a fatality rate around 10%. Today, urinary antigen tests (UATs) detecting Legionella pneumophila serogroup 1 (Lp sg 1) lipopolysaccharide (LPS) account for 70-80% of LD diagnosis in Europe and United States. These methods, based on immunochromatographic (ICT), immunofluorimetric (IFT) lateral flow assays or enzyme immunoassays (DA) are wildely used in European labs. Many tests are commercialized, with overall sensitivity of 70-90% and specificity >95%. Some works showed different performances when comparing one to another. However, no study has been conducted to compare a large panel of UATs on the same samples .
AIMS
Detection comparison of 9 Legionella pneumophila serogroup 1 (Lpl) LPS subgroups in surcharged urine samples (US) by 16 UATs Cross-reactivity evaluation with Lp sg 2-14 LPS This work is part of a global study, led by ESGLI, aiming to compare the performance of 16 UATs on US in 9 European National Reference Centers for Legionella.
METHOD
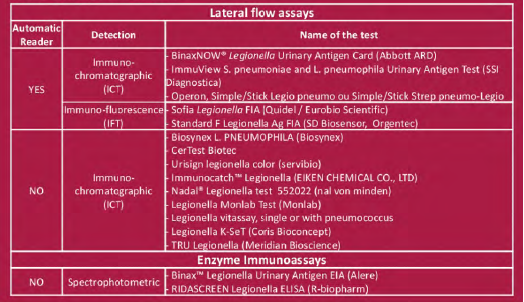
LPS were chemically extracted from reference Lpl Pontiac and non-Pontiac strains: Pontiac: Philadelphia, Benidorm, Knoxville and France/Allentown non-Pontiac: OLDA, Oxford, Heysham, Bellingham and Camperdown LPS from Lp2-14 were also purified Fixed amounts of LPS between 0.03 ng/mL and 30 ng/mL for Lp1 and 3, 30 or 3000 ng/mL for Lp2-14 were added to a pool of sterile urine (US) that tested negative with the 16 UATs. The tested concentration were chosen according to published data (1). LPS concentration was defined by 2-keto-3-deoxyoctonate (KDO) determination. They were assessed in one run or triplicates Tested UATs are listed on Table
RESULTS
AUTOMATIC vs OPTICAL READING
Results for 0.03 ng/mL LPS concentration
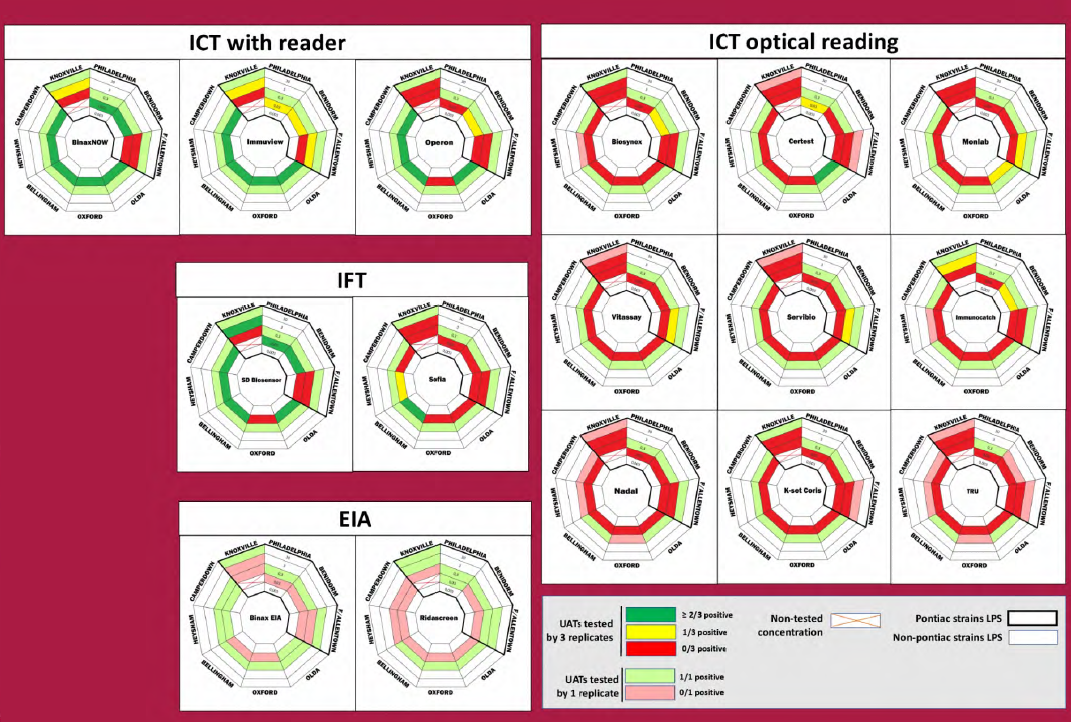
• Detection of 5/8 LPS by 4/5 ICT or IFT with reader (Binaw now, Immuview, Operon and SD Biosensor)
• Detection of ? 1/8 LPS by 3/9 ICT with optical reading(Certest, Monlab and Immunocatch)
• No detection by 7/8 ICT with optical reading (Biosynex Vitassay Servibio Nadal, K-set Coris, Tru)
–>4 better performance for UAT with automatic reader
ICT and IFT vs EIA
Results for 0.03 ng/mL LPS concentration
• Detection of ?. 1/8 LPS by 2/2 EIA
–> EIA not more sensitive than ICT with reader
PONTIAC VS NON-PONTIAC LPS DETECTION
Results for 0.3 ng/rnL and 0.03 LPS concentration
• Detection by 13/16 UATs of 4/5 non-Pontiac strains at 0.3 ng/mL (4/16 for 0.03 ng/mL concentration)
• Detection by 15/16 UATs of 2/4 Pontiac strains at 0.3 ng/mL (2/16 for 0.03 ng/mL concentration) .14
–> non-Pontiac LPS are well detected as previously shown (1)
–> Philadephila and Benidorm better detection among Pontiac LPS
Lp sg 2 —14 CROSS REACTIVITY
Only 4 LPS were detected at 30 ng/mL concentration:
– Lp7 was detected by 10/16 UATs Lp12 was deteted by 4/16 UATs
– Lp9 was detected by 2/16 UATs Lp4 was detected by 1/16 UATs
Other Lp2-14 LPS were detected at 3000 ng/mL concentration or non detected
CONCLUSIONS
This study describes an easy method based on extracted LPS to compare UATs. ICTs with a reader were more sensitive, with the best sensitivity obtained for ICT with reader and one IFT. The two ELISA kits didn’t provide a gain of sensitivity. Non-Pontiac LPS are globally detected at lower concentration than Pontiac LPS. Only 4 Lp2-14 LPS were detected at a concentration 100 times higher than Lpl subgroups.
However, our results do not reflect the real LPS concentration in clinical US. That limitation should be acknowledged with the comparison of the 15 UATs in LD patients US.